Breast Implant Illness
Breast implant illness or BII
Incidence of Breast Implant Illness (BII)
BII has been estimated to develop in between one and 10% of implanted patients. But the truth of the matter is that no one really knows what the incidence is. Some argue that ALL patients with breast implants will develop BII given enough time.
Etiology of Breast Implant Illness
Although the etiology of BII is unclear, it is felt to be the result of chronic reaction and inflammation to either silicone or other chemicals in the processing of breast implants.
Symptoms of Breast Implant Illness
Symptoms are varied and span from head to toe: hair loss, brain fog / difficulty concentrating, headaches, insomnia, dry eyes and mouth, skin rashes, difficulty breathing, muscle pain and weakness, bone pain, joint pain, irritable bowel, frequent infections. Severe cases can progress to autoimmune diseases. BII sceptics will often argue that many of these symptoms are the same symptoms one might see with menopause or that the symptoms can be just about anything. Nevertheless, these symptoms are consistently inflammatory in nature. The 5 most common symptoms are fatigue, brain fog, joint pain, muscle pain and skin rashes, in that order.
Important
Given all the talk about BII on the internet and social media, many implant patients consult Dr. Nicolaidis, worried and sometimes even anxious about developing breast implant illness.
Do not forget that not all implanted patients develop BII. Breast, neck, back and shoulder pain are mechanical problems due to the additional weight over the chest, not BII.
Also, breast implants cannot be blamed for every problem a patient has; to that end, it is important that patients see their family doctor first in order to rule out a serious illness. The symptoms with BII are fairly consistent. Dr. Nicolaidis will assess at the time of consultation.
Risk factors
Patients at higher risk of developing BII with breast implants are THOUGHT to be those with the following: severe allergy history; skin disorders; thyroid disease; asthma; history of autoimmune disease. But this remains speculation for now. Dr. Nicolaidis decided to stop posing breast implants after meeting numerous patients who developed BII despite having none of these supposed risk factors.
Proof of Breast Implant Illness
Why Breast Implant Illness (BII) Remains Controversial
So why did the FDA withdraw gel implants in 1992, only to allow their return in 2006? As mentioned earlier, there were numerous studies suggesting breast implant safety while other studies suggested the opposite. In 2020, Dr. Nicolaidis approached the Department of Rheumatology at University of Montreal in order to consider the issue once again.
A Systematic Review Conducted at the University of Montreal
Under Dr. Sabrina Hoa, a systematic literature review of all published studies on the association between silicone breast implants and rheumatic disorders was performed. When we focused on just scleroderma (one of the most common rheumatic disorders) and excluded all studies with either conflict of interest, small patient numbers or poor study methods, only two good studies remained…
The Most Reliable Studies on Breast Implant Illness
Fryzek in 2007 looked at the Danish National Hospital Register, finding that patients with breast implants had a three times higher incidence of scleroderma. In 2018, Watad did a cross sectional analysis using the Israeli Healthcare Database, comparing 24,651 women with silicone breast implants to 98,604 matched women without implants and found that women with implants had a significantly higher incidence of autoimmune or rheumatic disorders.
The Ongoing Problem of Conflicts of Interest
To the disappointment of both Dr. Nicolaidis and the BII community, CONFLICT OF INTEREST continues to be a problem with respect to breast implant safety. In 2020-21, the Aesthetic Society Education and Research Foundation (ASERF) gave significant funding for a prospective study of BII and capsulectomies as treatment. Unfortunately, both lead researchers were consultants for breast implant companies. Given the obvious conflict of interest, the validity of their results were questioned even before completion of the study.
Major Flaws in the ASERF Study
Moreover, the study had 4 MAJOR flaws:
- The BII and non-BII patients had completely different implant types; the implants in both groups SHOULD HAVE BEEN the same in order to compare the 2 groups properly.
- The type of capsulectomy for each patient was decided by surgeon and patient; the type of capsulectomy for each patient SHOULD HAVE BEEN assigned randomly.
- The number of patients was small and therefore the resulting statistical significance was poor.
- The researchers chose not to analyze silicone levels in the groups; rather, they decided to focus on heavy metals.
60 years of problems with silicone implants but they did not study silicone levels in depth.
For these reasons, we consider the conclusions of the ASERF study to be essentially irrelevant for the care of BII patients.
Clinical Improvements After Explantation
Despite that, multiple RETROSPECTIVE studies have now shown an improvement in symptoms following explantation, such that even the Plastic Surgeon nonbelievers acknowledge this improvement. In fact, even the flawed ASERF study mentioned above confirmed symptom improvement following explantation.
Evidence Beyond the Placebo Effect
Nevertheless, the sceptics argue that this symptom improvement after explantation is simply placebo effect. Dr. Nicolaidis presented the first PROSPECTIVE study of 182 consecutive explant patients at both the American Society of Plastic Surgeons Meeting and the Breast Implant Health Summit in the Fall of 2020. Symptoms that improved the most following explantation were respiratory problems (89% improvement), fatigue (74%), muscle pain and weakness (70%) and brain fog (66%). For the sceptics who might argue that this is all placebo effect, Dr. Nicolaidis found that two patients stopped their bronchodilators altogether after explantation, three stopped Synthroid, two decreased their Synthroid dose, two decreased their doses of gastrointestinal medications, one stopped their blood pressure medication, 6 stopped pain medications and 12 others either reduced or stopped other medications. Dr. J. W. Cohen Tervaert, a Rheumatologist at University of Alberta, has found that patients who develop established autoimmune disease with breast implants require not only capsulectomy but additional medical treatment as well for symptom improvement (discussed later).
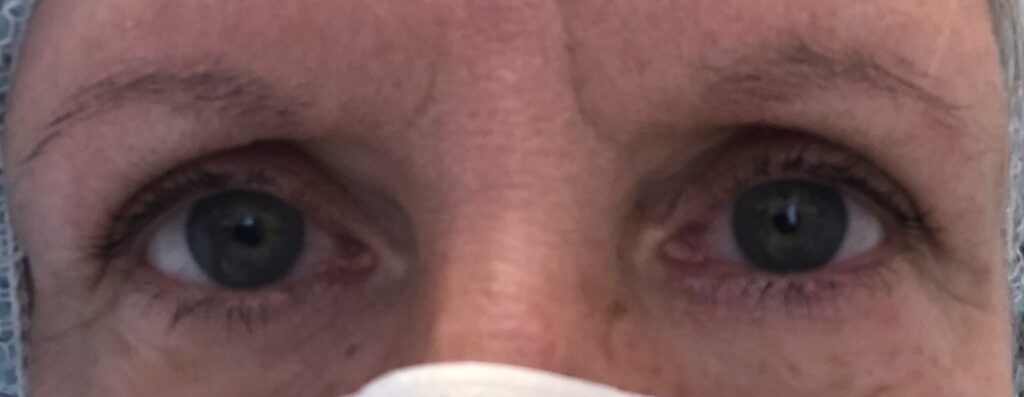
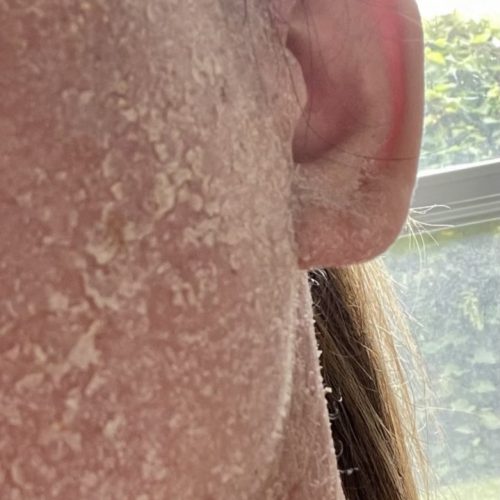
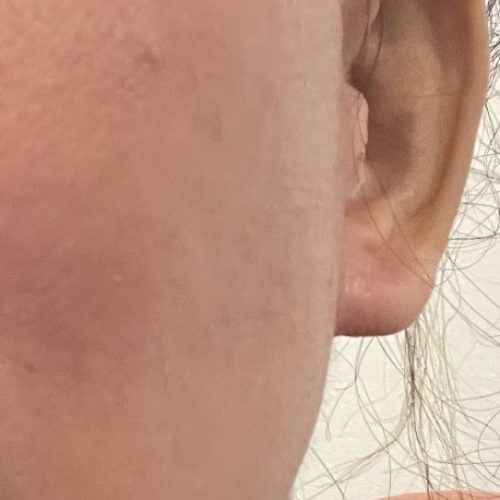
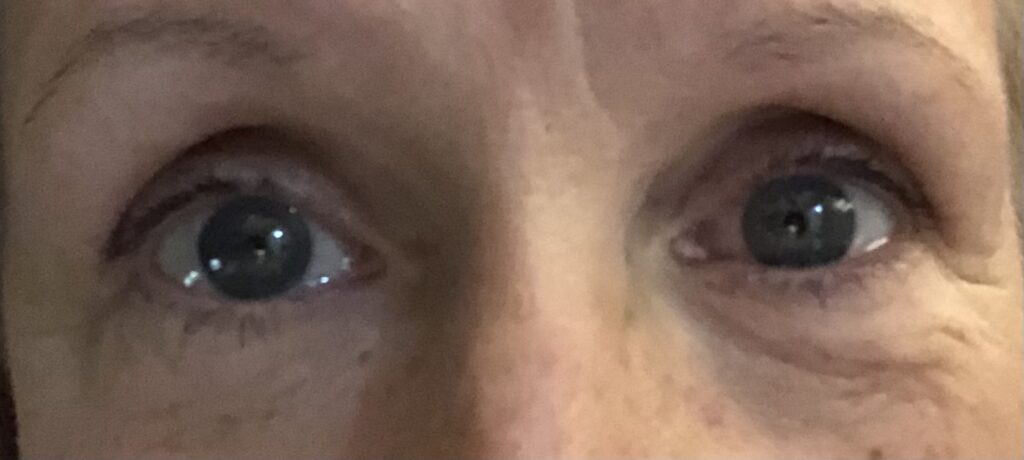 Dry eyes resolved three weeks after explantation
Dry eyes resolved three weeks after explantation
*Results are not guaranteed.*

